Thromboelastography and rotational thromboelastometry in bleeding patients with coagulopathy
Published 2020
Citation: J Trauma. 89(6):999-1017, December 2020
Authors
Bugaev, Nikolay MD; Como, John J. MD, MPH; Golani, Guy MD; Freeman, Jennifer J. MD; Sawhney, Jaswin S. MD; Vatsaas, Cory J. MD; Yorkgitis, Brian K. DO; Kreiner, Laura A. MD; Garcia, Nicole M. MD; Aziz, Hiba Abdel MD, FACS; Pappas, Peter A. MD; Mahoney, Eric J. MD; Brown, Zachary W. DO; Kasotakis, George MD, MPH
Author Information
From the Division of Trauma and Acute Care Surgery (N.B.), Tufts Medical Center, Tufts University School of Medicine, Boston, Massachusetts; Department of Surgery (J.J.C.), MetroHealth Medical Center, Cleveland, Ohio; Department of Surgery A (G.G.), Soroka Medical Center, Beer Sheva, Israel; Department of Surgery (J.J.F.), TCU and UNTHSC School of Medicine, Fort Worth, Texas; Maine Medical Center (J.S.S.), Portland, Maine; Division of Trauma and Critical Care Surgery (C.J.V.), Department of Surgery, Duke University School of Medicine, Durham, North Carolina; University of Florida College of Medicine—Jacksonville (B.K.Y.), Jacksonville, Florida; Case Western University School of Medicine (L.A.K.), Cleveland, Ohio; Division of Trauma, Surgical Critical Care, and Acute Care Surgery (N.M.G.), Department of Surgery, Brody School of Medicine, East Carolina University, Greenville; Weill Cornell University (H.A.A.), Doha, Qatar; Department of Surgery (P.A.P.), College of Medicine, University of Central Florida, Orlando; Division of Trauma and Acute Care Surgery (E.J.M.), Tufts Medical Center, Tufts University School of Medicine, Boston, Massachusetts; 2D Medical Battalion, 2D Marine Logistics Group (Z.W.B.), Camp LeJeune, North Carolina; Department of Surgery (Z.W.B.), Uniformed Services University of the Health Sciences, Bethesda; and Division of Trauma and Critical Care Surgery (G.K.), Department of Surgery, Duke University School of Medicine, Durham, North Carolina.
Submitted: July 21, 2020, Accepted: August 31, 2020, Published online: September 16, 2020.
The study was presented at the 33nd EAST Annual Scientific Assembly, January 17, 2020, in Orlando, Florida.
Supplemental digital content is available for this article. Direct URL citations appear in the printed text, and links to the digital files are provided in the HTML text of this article on the journal’s Web site (www.jtrauma.com).
Address for reprints: Nikolay Bugaev, MD, Division of Trauma and Acute Care Surgery, Tufts Medical Center, Tufts University School of Medicine, 800 Washington St, #4488, Boston, MA 02111; email: nbugaev@tuftsmedicalcenter.org.
Abstract
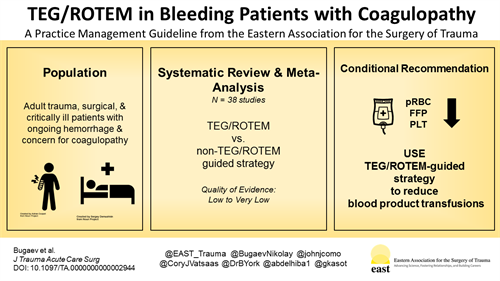
BACKGROUND
Assessment of the immediate need for specific blood product transfusions in acutely bleeding patients is challenging. Clinical assessment and commonly used coagulation tests are inaccurate and time-consuming. The goal of this practice management guideline was to evaluate the role of the viscoelasticity tests, which are thromboelastography (TEG) and rotational thromboelastometry (ROTEM), in the management of acutely bleeding trauma, surgical, and critically ill patients.
METHODS
Systematic review and meta-analyses of manuscripts comparing TEG/ROTEM with non–TEG/ROTEM-guided blood products transfusions strategies were performed. The Grading of Recommendations Assessment, Development and Evaluation methodology was applied to assess the level of evidence and create recommendations for TEG/ROTEM-guided blood product transfusions in adult trauma, surgical, and critically ill patients.
RESULTS
Using TEG/ROTEM-guided blood transfusions in acutely bleeding trauma, surgical, and critically ill patients was associated with a tendency to fewer blood product transfusions in all populations. Thromboelastography/ROTEM-guided transfusions were associated with a reduced number of additional invasive hemostatic interventions (angioembolic, endoscopic, or surgical) in surgical patients. Thromboelastography/ROTEM-guided transfusions were associated with a reduction in mortality in trauma patients.
CONCLUSION
In patients with ongoing hemorrhage and concern for coagulopathy, we conditionally recommend using TEG/ROTEM-guided transfusions, compared with traditional coagulation parameters, to guide blood component transfusions in each of the following three groups: adult trauma patients, adult surgical patients, and adult patients with critical illness.
LEVEL OF EVIDENCE
Systematic Review/Meta-Analysis, level III.
Management of acutely bleeding patients consists of definitive control of the bleeding source, restoration of blood volume, and correction of any associated coagulopathy. The assessment of the coagulopathy and prediction of blood component transfusion requirements in patients with ongoing hemorrhage in real time are challenging.[1] The standard coagulation assays commonly used in clinical practice, which are prothrombin time (PT), international normalized ratio (INR), activated partial prothrombin time (PTT), platelet count (PLT), and fibrinogen (Fibr), frequently provide inadequate information about clinically significant coagulopathy and the degree of blood loss.[2–4] These assays were originally created to assess coagulation profiles in patients with inherent deficiency of coagulation factors, not in patients with acute bleeding.[1][5] These tests are frequently inaccurate in predicting blood component transfusion needs and do not accurately reflect coagulopathy in patients with hypothermia and acidosis.[1]
In contrast to the routine coagulation assays, thromboelastography (TEG) and rotational thromboelastometry (ROTEM) assess viscoelastic clot strength in real time as an ongoing process rather than reflecting individual steps of the coagulation cascade.[6–8] In addition, TEG/ROTEM can detect the timing and extent of fibrinolysis, which is not accurately estimated by standard coagulation tests.[9]
The most recent Cochrane systematic review analyzed 17 randomized controlled trials (RCTs) that evaluated utilization of TEG/ROTEM-guided blood product transfusions in adult and pediatric populations.[10] The authors concluded that TEG/ROTEM-guided resuscitation may reduce both the transfusion of blood products and associated morbidity.
Taking into consideration the growing interest in the usage of viscoelastic methods in various types of surgical patients, the Eastern Association for the Surgery of Trauma Practice Management Guidelines Committee aimed to formulate recommendations regarding TEG/ROTEM-guided blood product transfusions in adult trauma, surgical, and critically ill patients with ongoing bleeding.
Objectives
The objective of this review was to evaluate outcomes in acutely bleeding adult trauma, surgical, and critically ill patients with concern for significant coagulopathy in whom either TEG or ROTEM (TEG/ROTEM) was used to guide blood product transfusions and compare them with patients in whom no TEG/ROTEM was used to guide transfusions. The Grading of Recommendations Assessment, Development and Evaluation (GRADE) methodology was used to assess the level of existing evidence and create recommendations.[11] The Eastern Association for the Surgery of Trauma working group performed a systematic review and meta-analysis of the relevant clinical studies.
The following Population, Intervention, Comparison, Outcomes (PICO) questions were developed by the working group:
1. PICO 1
In adult trauma patients with ongoing hemorrhage and concern for coagulopathy (P), should a TEG/ROTEM-guided transfusion strategy (I) versus a non-TEG/ROTEM transfusion strategy (C) be used to reduce mortality, blood product transfusions, and the need for additional hemostatic (angioembolic, endoscopic, or surgical) interventions (O)?
2. PICO 2
In adult surgical patients with ongoing hemorrhage and concern for coagulopathy (P), should a TEG/ROTEM-guided transfusion strategy (I) versus a non-TEG/ROTEM transfusion strategy (C) be used to reduce mortality, blood product transfusions, and the need for additional hemostatic (angioembolic, endoscopic, or surgical) interventions (O)?
3. PICO 3
In adult critically ill patients with ongoing hemorrhage and concern for coagulopathy (P), should a TEG/ROTEM-guided transfusion strategy (I) versus a non-TEG/ROTEM transfusion strategy (C) be used to reduce mortality, blood product transfusions, and the need for additional hemostatic (angioembolic, endoscopic, or surgical) interventions (O)?
Outcome Measure Type
The outcomes were proposed and independently rated by each group member on a scale of 1 to 9, and the median score for each outcome was calculated and assigned as the final score.
Outcomes scored between 7 and 9 were considered critical and included the following: transfusion of packed red blood cells (PRBCs); transfusion of fresh frozen plasma (FFP); transfusion of PLT, cryoprecipitate (Cryo), Fibr, and prothrombin complex concentrate (PCC); need for additional hemostatic interventions (angioembolic, endoscopic, or surgical); time to bleeding control; and mortality.
Transfusion of PRBC, FFP, PLT, Cryo, Fibr, and PCC was reported as the number of units and volume of the transfused product and the number of patients being transfused. To simplify the reporting of the results, all these outcomes were combined into one: “need for blood product transfusion.”
Time to bleeding control was not reported in any of the included studies, so this outcome was excluded.
Identification of References
A professional medical librarian (J.R.) performed a search of citations in the following databases: PubMed, Embase, Cochrane Library, Web of Science, and Ovid Medline. The search was performed using the following Medical Subjects Headings (MeSH) terms: “hemorrhage,” “blood loss,” “bleeding,” “thromboelastography,” “thromboelastograph,” “thromboelastometry,” “ROTEM,” and “TEG” (Supplemental Digital Content, Appendix 1, http://links.lww.com/TA/B804). The original literature search included articles published between January 1, 1946, and June 2019. This was subsequently updated in June 2020, to assure inclusion of the newest literature.
Randomized controlled trials and both prospective and retrospective clinical studies in adults (age, ≥18 years) were considered for inclusion. Review articles, meta-analyses, case reports, case series without a comparison group, and non-English language publications were excluded.
Each title and abstract was screened independently by two members of the working group, with irrelevant studies being discarded. Then, the full texts of the remaining articles were independently screened by two independent working group members. Selected studies were included for final data extraction and analysis. All disagreements between the reviewers were adjudicated by discussion and consensus among the individuals. When consensus was not reached, a third reviewer was involved as an arbitrator (Fig. 1).
Data Extraction and Methodology
A total of 38 studies were included.[12–49] Data extraction was performed independently by two team members for each of the selected studies and entered into a Microsoft Excel 2013 (Redmond, WA) spreadsheet. The meta-analysis and creation of forest plots were performed using Review Manager (RevMan) (version 5.3; Cochrane Collaboration, Oxford, United Kingdom). Dichotomous outcomes were reported as risk ratio (RR). Continuous variables were reported as only standardized mean difference (SMD), because the outcomes of interest in the included studies were reported in different ways: units of blood product per patient and volume of transfused blood product per patient. Also, the varying definitions of PLTs, PCC, and Cryo units transfused precluded from performing calculations of mean difference instead of SMD. In the studies where the continuous data were presented as medians and interquartile ranges, means and standard deviations were calculated according the Cochrane Database Systematic Review recommendations.[50] Confidence interval (CI) of 95% was presented with RR and SMD.
The absolute effect (AE) of the TEG/ROTEM was reported for dichotomous outcomes where the beneficial effect of the intervention was demonstrated. The AE was calculated using the GRADEpro Guideline Development Tool (McMaster University, Hamilton Ontario Canada; Evidence Prime Inc. Hamilton, Ontario, Canada).[51] In general, the calculations of the AE take into consideration the baseline risk and relative effect size, and use the results of the meta-analyses for this purpose. For dichotomous outcomes, the AE was reported as a number of patients with the outcome after the exposure to TEG/ROTEM per 1,000 patients. Confidence interval of 95% was presented for the AE as well.
There were no AE presented for continuous outcomes, since they all were reported in different ways in the included studies and only the relative effect of the intervention was calculated.
Grading the Evidence
The available evidence was assessed according to the GRADE methodology as high, moderate, low, or very low quality. The quality of evidence was downgraded for observational studies, presence of bias, inconsistency, indirectness, and imprecision. Risk of bias was assessed in six domains: sequence generation, allocation concealment, blinding of outcome assessment, incomplete outcome data, selective outcome reporting, and “other issues” (Supplemental Digital Content, Appendix 2, http://links.lww.com/TA/B805).
Results for the Use of TEG/ROTEM in Trauma Patients (PICO 1)
In adult trauma patients with ongoing hemorrhage and concern for coagulopathy (P), should a TEG/ROTEM-guided transfusion strategy (I) versus a non-TEG/ROTEM-guided strategy (C) be used to reduce mortality, blood product transfusions, and the need for additional hemostatic (angioembolic, endoscopic, or surgical) interventions (O)?
Qualitative Analysis
A total of seven studies[12–18] were selected to answer PICO 1: two RCTs,[12][16] one retrospective with a control group,[13] one prospective with a historical control groups,[14] two retrospective before and after,[15][18] and one retrospective with a control group from a national trauma database.[17] The included studies contained 481 patients in the intervention group and 1,224 in the control groups. All included studies reported utilization of TEG/ROTEM based on local institutional protocols. The indications to use TEG/ROTEM differed in the included studies. Some of these indications included patients requiring MTP activation,[12][18] any blood product transfusions,[13] severely injured patients with Injury Severity Score of >15 who required blood transfusions,[14][15][17] and patients with burns.[16] Most of the studies evaluated ROTEM,[13–18] and only one study evaluated TEG.[12] The intervention (TEG/ROTEM) was compared with standard coagulation assays (PT, PTT, INR, Fibr)[12–14][16–18] and treating physician clinical assessment.[13][15][16]
All but one[12] of the included studies showed no difference in 24 hours[13][14] and hospital mortality.[13–18] Gonzalez et al.[12] demonstrated reduced mortality in those patients in whom MTP therapy was guided by TEG (19.6% vs. 36.4%, p < 0.05). Utilization of TEG/ROTEM in comparison with non-TEG/ROTEM approach had an overall inconsistent effect on blood product usage, leading to either fewer transfusions of blood products including PRBC,[13] FFP,[13][14][16] PLT,[13–15] and Cryo,[13][15] or no effect of TEG/ROTEM-guided transfusions on the amount of transfused PRBCs,[12][14–17] FFP,[15][18] PLT,[12][18] Cryo.[12][18] An increase in the usage of Fibr was reported in one study.[13]
The need for additional angioembolic, endoscopic, or surgical intervention to address ongoing bleeding was not reported in any of the included studies.
Quantitative Analysis (Meta-analysis)
Data from all included studies were suitable for meta-analysis. There was a beneficial effect of TEG/ROTEM usage on number of patients transfused with PRBCs (RR, 0.74; 95% CI, 0.67–0.82; AE, 251 patients fewer; 95% CI, from 319 fewer to 174 fewer per 1,000 patients), number of patients transfused with PLTs (RR, 0.35; 95% CI, 0.22–0.55; AE, 289 patients fewer; 95% CI, from 346 fewer to 200 fewer per 1,000 patients), and number of transfused PRBC units (SMD, –0.38; 95% CI, –0.64 to −0.12) as well as mortality (RR, 0.75; 95% CI, 0.59–0.95; AE, 38 patients fewer; 95% CI, from 62 fewer to 8 fewer per 1,000 patients) (Table 1A, Fig. 2).
| Evidence Table 1A: PICO 1 TEG/ROTEM in Trauma Patients | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
Certainty assessment |
No. Patients |
Effect |
Certainty |
Importance |
||||||||
|
No. Studies |
Study Design |
Risk of bias |
Inconsistency |
Indirectness |
Imprecision |
Other Considerations |
TEG/ROTEM |
No TEG/ROTEM |
Relative (95% CI) |
Absolute (95% CI) |
||
|
No. patients transfused with PRBC |
||||||||||||
|
2 |
Observational studies[13][17] |
Not serious |
Not serious |
Not serious |
Not serious |
None |
129/182 (70.9%) |
679/703 (96.6%) |
RR, 0.74 (0.67 to 0.82) |
251 fewer per 1,000 (from 319 fewer to 174 fewer) |
XXOO Low |
Critical |
|
No. transfused PRBC units/patient |
||||||||||||
|
7 |
Observational studies[12–18] |
Not serious |
Serious** |
Not serious |
Serious* |
None |
480 |
979 |
— |
SMD, 0.38; SD, fewer (0.64 fewer to 0.12 fewer) |
XOOO Very low |
Critical |
|
No. patients transfused with PLT |
||||||||||||
|
3 |
Observational studies[13][17][18] |
Not serious |
Not serious |
Not serious |
Not serious |
None |
41/229 (17.9%) |
303/703 (44.4%) |
RR, 0.35 (0.22–0.55) |
297 fewer per 1,000 (from 346 fewer to 200 fewer) |
XXOO Low |
Critical |
|
No. transfused PLTs units/patient |
||||||||||||
|
3 |
Observational studies[12][14][18] |
Not serious |
Serious** |
Not serious |
Serious* |
None |
199 |
205 |
— |
SMD, 0.21; SD, fewer (0.41 fewer to 0.01 more) |
XOOO Very low |
Critical |
|
No. transfused FFP units/patient |
||||||||||||
|
5 |
Observational studies[12–15][18] |
Not serious |
Serious** |
Not serious |
Serious* |
None |
386 |
441 |
— |
SMD, 0.29; SD, fewer (0.91 fewer to 0.34 more) |
XOOO Very low |
Critical |
|
Mortality |
||||||||||||
|
6 |
Observational studies[12–15][17][18] |
Not serious |
Serious** |
Not serious |
Serious* |
None |
82/466 (17.6%) |
158/1,042 (15.2%) |
RR, 0.75 (0.59–0.95) |
38 fewer per 1,000 (from 62 fewer to 8 fewer) |
XOOO Very low |
Critical |
| View/Download Table 1 as image | ||||||||||||
| Evidence Table 1B. PICO 2 TEG/ROTEM in Surgical Patients | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
Certainty Assessment |
No. Patients |
Effect |
||||||||||
|
No. Studies |
Study Design |
Risk of Bias |
Inconsistency |
Indirectness |
Imprecision |
Other Considerations |
TEG/ROTEM (Intervention) |
(No TEG/ROTEM) |
Relative (95% CI) |
Absolute (95% CI) |
Certainty |
Importance |
|
No. patients transfused with PRBC |
||||||||||||
|
14[19][21–23][25][28–31][33][35][37–39] |
Observational studies |
Not serious |
Not serious |
Not serious |
Serious * |
None |
1,889/3,721 (50.8%) |
2,025/3,234 (62.6%) |
RR, 0.83 (0.79–0.88) |
106 fewer per 1,000 (from 131 fewer to 75 fewer) |
XOOO Very low |
Critical |
|
No. transfused PRBC units |
||||||||||||
|
15[19–25][27–29][32][34][35][38][39] |
Observational studies |
Not serious |
Not serious |
Not serious |
Serious* |
None |
3,155 |
2,764 |
— |
SMD, 0.35; SD, lower (0.66 lower to 0.04 lower) |
XOOO Very low |
Critical |
|
No. patients transfused with FFP |
||||||||||||
|
14[19][21–23][25][28–31][33][35][37–39] |
Observational studies |
Not serious |
Serious** |
Not serious |
Serious* |
None |
449/3,721 (12.1%) |
1,118/3,305 (33.8%) |
RR, 0.42 (0.27–0.65) |
196fewer per 1,000 (from 247 fewer to 118 fewer) |
XOOO Very low |
Critical |
|
No. transfused FFP units/volume |
||||||||||||
|
14[19][21–25][27–29][32][34][35][38][39] |
Observational studies |
Not serious |
Not serious |
Not serious |
Serious* |
None |
3,130 |
3,031 |
— |
SMD, 0.58; SD, lower (0.93 lower to 0.24 lower) |
XOOO Very low |
Critical |
|
No. patients transfused with PLT |
||||||||||||
|
13[18][20–22][24][27–31][34][38][39] |
Observational studies |
Not serious |
Serious** |
Not serious |
Serious* |
None |
871/3,622 (24.0%) |
933/3,140 (29.7%) |
RR, 0.82 (0.67–1.01) |
53fewer per 1,000 (from 98 fewer to 3 fewer) |
XOOO Very low |
Critical |
|
No. transfused PLT units |
||||||||||||
|
10[21–25][27–29][34][39] |
Observational studies |
Not serious |
Serious** |
Not serious |
Very serious* |
None |
2,850 |
2,463 |
— |
MD 0.02 fewer (0.07 fewer to 0.03 more) |
XOOO Very low |
Critical |
|
No. Patients transfused with Cryo |
||||||||||||
|
5[25][29–31][39] |
Observational studies |
Not serious |
Serious** |
Not serious |
Serious* |
None |
144/1,063 (13.5%) |
230/1,007 (22.8%) |
RR, 0.88 (0.36–2.16) |
27fewer per 1,000 (from 146 fewer to 265 more) |
XOOO Very low |
Critical |
|
No. transfused Cryo units |
||||||||||||
|
4[25][29][34][39] |
Observational studies |
Not serious |
Serious** |
Not serious |
Serious* |
None |
440 |
486 |
— |
SMD, 0.05; SD, lower (0.86 lower to 0.96 higher) |
XOOO Very low |
Critical |
|
No. patients transfused with Fibr |
||||||||||||
|
5[21][22][33][35][38] |
Observational studies |
Not serious |
Serious** |
Not serious |
Serious* |
None |
311/2,341 (13.3%) |
121/1,914 (6.3%) |
RR, 2.36 (0.93–6.00) |
86more per 1,000 (from 4 fewer to 316 more) |
XOOO Very low |
Critical |
|
Fibr transfused in grams/patient |
||||||||||||
|
6[20–22][27][38][39] |
Observational studies |
Not serious |
Serious** |
Not serious |
Serious* |
None |
2,398 |
1,954 |
— |
SMD, 1.0; SD, higher (0.27 lower to 1.72 higher) |
XOOO Very low |
Critical |
|
No. patients transfused with PCC |
||||||||||||
|
4[21][22][35][39] |
Observational studies |
Not serious |
Serious** |
Not serious |
Serious* |
None |
235/2,306 (10.2%) |
125/1,868 (6.7%) |
RR, 1.02 (0.38–2.77) |
1 more per 1,000 (from 41 fewer to 118 more) |
XOOO Very low |
Critical |
|
No. transfused PCC units |
||||||||||||
|
4[21][22][35][39] |
Observational studies |
Not serious |
Serious** |
Not serious |
Serious* |
None |
2,306 |
1,868 |
— |
SMD, 0.37; SD, lower (1.08 lower to 0.34 higher) |
XOOO Very low |
Critical |
|
Reoperation |
||||||||||||
|
12[19–25][30][31][35][38][39] |
Observational studies |
Not serious |
Serious** |
Not serious |
Serious* |
None |
100/3,612 (2.8%) |
166/3,126 (5.2%) |
RR, 0.55 (0.43–0.70) |
24 fewer per 1,000 (from 30 fewer to 16 fewer) |
XOOO Very low |
Critical |
|
Mortality |
||||||||||||
|
9[19][21–23][25][27][29][30][38] |
Observational studies |
Not serious |
Serious** |
Not serious |
Serious* |
None |
137/2,973 (4.6%) |
122/2,489 (4.9%) |
RR, 0.96 (0.75–1.22) |
2 fewer per 1,000 (from 12 fewer to 11 more) |
XOOO Very low |
Critical |
| View/Download Table 1 as image | ||||||||||||

Figure 2. PICO 1. (A), Number of patients transfused with PRBC. (B), Number of transfused PRBC units per patient. (C), Number of patients transfused with PLT. (D), Number of transfused PLTs units per patient. (E), Number of transfused FFP units per patient. (F), Mortality.
There was no beneficial effect of TEG/ROTEM utilization on the number of transfused units of FFPs and PLTs.
Grading the Evidence
The evidence was assessed applying the GRADE framework (Table 1A; Supplemental Digital Content, Appendix 2, http://links.lww.com/TA/B805). First, the level of evidence was downgraded for all outcomes because of the inclusion of observational studies. We also downgraded the level of evidence because of the inconsistent effect of TEG/ROTEM on blood product transfusions. The low number of included subjects and wide CIs led to downgrading the level of evidence for imprecision in all outcomes. Overall, the level of evidence was assessed as very low.
Recommendations for the Use TEG/ROTEM in Trauma Patients (PICO 1)
Based on the analysis of included studies, the effect of TEG/ROTEM on the selected outcomes, and the very low level of evidence, we conditionally recommend using TEG/ROTEM-guided strategy versus non–TEG/ROTEM-guided strategy in adult trauma patients with ongoing hemorrhage and concern for coagulopathy to reduce blood product transfusions and mortality (Table 2). Although the effect of TEG/ROTEM was inconsistent across the selected outcomes, the potential benefit of fewer patients being exposed to blood products and reduced mortality, combined with the lack of harm to the patient from using TEG/ROTEM, led us to make this conditional recommendation.
| TABLE 2A - Recommendations | |
|---|---|
|
PICOs |
Recommendations |
|
TEG/ROTEM-guided transfusion strategy in adult trauma patients with ongoing hemorrhage and concern for coagulopathy |
We conditionally recommend using TEG/ROTEM-guided strategy vs. non–TEG/ROTEM-guided strategy in adult trauma patients with ongoing hemorrhage and concern for coagulopathy to reduce blood product transfusions. |
|
TEG/ROTEM-guided transfusion strategy in adult surgical patients with ongoing hemorrhage and concern for coagulopathy |
We conditionally recommend using a TEG/ROTEM-guided strategy vs. a non–TEG/ROTEM-guided strategy in adult surgical patients with ongoing hemorrhage and concern for coagulopathy, to reduce blood product transfusions. |
|
TEG/ROTEM-guided transfusion strategy in adult critically ill patients with ongoing hemorrhage and concern for coagulopathy |
We conditionally recommend using TEG/ROTEM-guided strategy vs. non–TEG/ROTEM-guided strategy in adult critically ill patients with ongoing hemorrhage and concern for coagulopathy to reduce blood product transfusions. |
| View/Download Table 2A as image | |
The need for additional angioembolic, endoscopic, or surgical interventions to address ongoing bleeding was not reported in any of the included studies, hence their lack of mention in the recommendation.
Results for the Use of TEG in Surgical Patients (PICO 2)
In adult surgical patients with ongoing hemorrhage and concern for coagulopathy (P), should a TEG/ROTEM-guided transfusion strategy (I) versus a non-TEG/ROTEM transfusion strategy (C) be used to reduce mortality, blood product transfusions, and the need for additional hemostatic (angioembolic, endoscopic, or surgical) interventions (O)?
Qualitative Analysis
Our search yielded a total of 21 studies: 8 RCTs,[19][21][24][26][28][34–36] 3 prospective studies with historical control groups,[23][29][38] 6 retrospective before-after studies,[25][30–33][37] and 3 retrospective reports with control groups.[20][22][27][39] The included studies contained 3,976 patients in the intervention group and 3,482 in the control group. All included studies reported utilization of TEG/ROTEM based on the institutional protocols. The selected studies included different populations: cardiac surgery patients,[19–26][28][31][33][35–38] general surgery/orthopedic surgery patients,[27] and patients who underwent liver[29][32][34][39] and lung transplantation.[30] Half the studies used TEG,[19][24–28][31][35][36] and the other half used ROTEM.[20–23][29][30][32–34][37–39] The intervention group (TEG/ROTEM) was compared with coagulation assays (PT, PTT, INR, Fibr) combined with clinical assessment,[19][21–35][38] coagulation assays alone,[20][26–41] or clinical assessment alone.[36][37]
Most reports showed no difference between TEG/ROTEM and non–TEG/ROTEM-guided transfusion strategies on reoperation rate,[19–21][23][24][28][30][33][35][38][39] while others demonstrated a benefit.[22][25][27][31] Utilization of TEG/ROTEM in comparison with a non-TEG/ROTEM approach had an overall inconsistent effect on blood product usage leading to either fewer transfusions of units of PRBC,[21–25][27][35][38] FFP,[19][21][23][24][27][29][34][35][38] PLT,[19][21][27][35] Cryo,[25] and PCC[21] or no difference in PRBC,[19][20][28][29][32][34][39] FFP,[22][28][32][39] PLT,[23–25][28][29][34][38][39] Cryo,[34][39] Fibr,[21][27][35][39] and PCC[22][35][39] transfusions.
Most of the reports showed that TEG/ROTEM-guided transfusions reduced the overall number of patients being transfused with PRBC,[22][25][30][31][33][35][37][38] FFP,[21][22][25][30][31][33][35][38][39] PLT,[19][21][25][30][31][35][39] Cryo,[25] and PCC.[21] At the same time, some studies showed no effect of TEG/ROTEM on the number of patients who required PRBC,[19][21][23][28][29][39] FFP,[19][23][28][29][37] PLT,[23][28][29][32][38] Cryo,[29–31][39] Fibr,[21][38] and PCC.[35][39]
The vast majority of the studies showed no difference in mortality between TEG/ROTEM and non-TEG/ROTEM patients.[19][21–30][33][34][38]
Quantitative Analysis (Meta-analysis)
All studies were suitable for meta-analysis (Table 1B, Fig. 3). There was a beneficial effect of TEG/ROTEM usage on number of patients transfused with PRBCs (RR, 0.83; 95% CI, 0.79–0.88; AE, 106 patients fewer; 95% CI, from 131 fewer to 75 fewer per 1,000 patients), on volume/units of transfused PRBCs (SMD, −0.35; 95% CI, −0.66 to −0.04), number of patients transfused with FFP (RR, 0.42; 95% CI, 0.27–0.65; AE, 196 patients fewer; 95% CI, from 247 fewer to 118 fewer per 1,000 patients), volume/units of transfused FFP (SMD, −0.58; 95% CI, –0.93 to −0.24), and on number of patients who required reoperation (RR, 0.55; 95% CI, 0.43–0.70; AE, 24 patients fewer; 95% CI, from 30 fewer to 16 fewer per 1,000 patients).

Figure 3A-B: PICO 2. (A), Number of patients transfused with PRBC. (B), Number of transfused PRBC units per volume.

Figure 3C-E: PICO 2. (C), Number of patients transfused with FFP. (D), Number of transfused FFP units per volume. (E), Number of patients transfused with PLT.

Figure 3F-I: PICO 2. (F), Number of transfused PLT units. (G), Number of patients transfused with Cryo. (H), Number of transfused Cryo units. (I), Number of patients transfused with Fibr.

Figure 3J-M: PICO 2. (J), Fibrinogen transfused in grams per patient. (K), Number of patients transfused with PCC. (L), Number of transfused PCC units. (M), The need for additional angioembolic, endoscopic, or surgical intervention.
PICO 2. (A), Number of patients transfused with PRBC. (B), Number of transfused PRBC units per volume. (C), Number of patients transfused with FFP. (D), Number of transfused FFP units per volume. (E), Number of patients transfused with PLT. (F), Number of transfused PLT units. (G), Number of patients transfused with Cryo. (H), Number of transfused Cryo units. (I), Number of patients transfused with Fibr. (J), Fibrinogen transfused in grams per patient. (K), Number of patients transfused with PCC. (L), Number of transfused PCC units. (M), The need for additional angioembolic, endoscopic, or surgical intervention. (N), Mortality.
There was no clear benefit of TEG/ROTEM on the transfusion of PLTs, PCC, Cryo, and Fibr (Fig. 3).
Grading the Evidence
The evidence was assessed applying the GRADE framework (Table 1B; Supplemental Digital Content, Appendix 2, http://links.lww.com/TA/B805). First, the level of evidence was downgraded for all outcomes because of the inclusion of observational studies. We also downgraded the level of evidence because of the inconsistent effect of TEG/ROTEM use on reoperation rate and blood transfusions. The level of evidence was further downgraded for wide CIs and the overall small number of subjects in the included studies. Overall, the level of evidence was assessed as very low.
Recommendations for the Use TEG in Surgical Patients (PICO 2)
Based on the analysis of included studies, the effect of TEG/ROTEM on the selected outcomes, and the very low level of evidence, we conditionally recommend using a TEG/ROTEM-guided strategy versus a non–TEG/ROTEM-guided strategy in adult surgical patients with ongoing hemorrhage and concern for coagulopathy, to reduce blood product transfusions (Table 2). Although the effect of TEG/ROTEM was inconsistent across the selected outcomes (blood transfusions, the need for additional angioembolic, endoscopic, or surgical intervention and mortality), the potential benefit from fewer patients exposed to blood transfusions and less blood product requirement, combined with no harm to the patient from using TEG/ROTEM, led us to make this conditional recommendation.
Results for the Use of TEG in Critically Ill Patients (PICO 3)
In adult critically ill patients with ongoing hemorrhage and concern for coagulopathy (P), should a TEG/ROTEM-guided transfusion strategy (I) versus a non-TEG/ROTEM transfusion strategy (C) be used to reduce mortality, blood product transfusions, and the need for additional hemostatic (angioembolic, endoscopic, or surgical) interventions (O)?
Qualitative Analysis
Our search yielded 10 studies: 3 RCTs,[42][43][49] 5 retrospective before-after studies,[40][41][45][47][48] and 2 retrospective studies with control groups.[43][46] The included studies contained 1,663 patients in the intervention group and 660 in control groups. All included studies reported utilization of TEG/ROTEM based on the institutional protocols. The selected studies included a heterogeneous population: cardiac surgery patients,[40–42] patients with upper gastrointestinal bleeding,[43] cirrhotic patients,[44][49] patients with postpartum hemorrhage,[45][47] patients with massive bleeding of various etiologies,[46] and critically ill patients who underwent minor surgical procedures in the intensive care unit.[48] Half the included studies used TEG,[41][42][44][46][49] and the other half used ROTEM.[40][43][45][47][48] The TEG/ROTEM was compared with traditional coagulation assays (PT, PTT, INR, Fibr) combined with clinical assessment,[40–42][46] coagulation assays alone,[44][48] or clinical assessment alone.[45][47][49]
Avidan et al.[42] performed two comparisons. First, in an RCT, TEG was compared with standard coagulation assays, and in a second, TEG was compared with a historical control group where the decision for blood transfusion was made based on the clinical impression of the treating physician. Overall, the results were in favor of TEG-guided transfusions versus clinical assessment alone. Comparisons with the standard coagulation essays showed no difference with TEG.
Utilization of TEG/ROTEM in comparison with the non-TEG/ROTEM approach had an overall inconsistent effect on blood components utilization leading to either fewer transfusions of units of PRBC,[40] FFP,[40][44–47] PLT,[40][44][45] Cryo,[44][45] or no difference in PRBC.[44][45] At the same time, some studies showed that the non-TEG/ROTEM strategy led to fewer transfusions of units of PRBC,[43][46] PLT,[40] and Cryo.[46]
Most of the reports showed that TEG/ROTEM-guided transfusions reduced the overall number of patients being transfused with PRBC,[40][47] FFP,[40][47][49] PLT,[41][47][49] and Cryo.[47]
The need for additional hemostatic interventions was not different in any of the reported studies.[40][42][43][45] Three studies reported mortality, with no apparent benefit of TEG/ROTEM.[43][44][46]
Quantitative Analysis (Meta-analysis)

Figure 4: PICO 3. (A), Number of patients transfused with PRBC. (B), Number of transfused PRBC units per volume. (C), Number of patients transfused with FFP. (D), Number of transfused FFP units per volume. (E), Number of patients transfused with PLT. (F), Number of transfused PLT units. (G), The need for additional angioembolic, endoscopic, or surgical intervention. (H), Mortality.
All 10 studies were suitable for meta-analysis (Table 1C, Fig. 4). There was a beneficial effect of TEG/ROTEM-guided transfusions on the number of patients transfused with PRBCs (RR, 0.83; 95% CI, 0.72–0.96; AE, 115 patients fewer; 95% CI, from 189 fewer to 27 fewer per 1,000 patients), the number of patients transfused FFPs (RR, 0.39; 95% CI, 0.17–0.93; AE, 127 patients fewer; 95% CI, from 173 fewer to 15 fewer).
Thromboelastography/ROTEM had no benefit on the number of patients transfused with PLTs; on volume/units of transfused PRBCs, FFPs, and PLTs; the need for additional hemostatic interventions; and mortality.
Grading the Evidence
The evidence was assessed applying the GRADE framework (Table 1C; Supplemental Digital Content, Appendix 2, http://links.lww.com/TA/B805). First, the level of evidence was downgraded for all outcomes because of the inclusion of observational studies. We also reduced the level of evidence because of the inconsistent effect of TEG/ROTEM on the transfusion of blood products. Since wide CIs were reported for blood product transfusion, reoperation, and mortality rate, and there was a small number of subjects in the included studies, the level of evidence was further downgraded for imprecision. Overall, the level of evidence was assessed to be very low.
Recommendations for the Use TEG in Critically Ill Patients (PICO 3)
Based on the analysis of included studies, the effect of TEG/ROTEM on the selected outcomes, and the very low level of evidence, we conditionally recommend using TEG/ROTEM-guided strategy versus non-TEG/ROTEM strategy in adult critically ill patients with ongoing hemorrhage and concern for coagulopathy to reduce blood product transfusions (Table 2). The results of the meta-analyses demonstrated that utilization of TEG/ROTEM reduced number of patients exposed to the transfusions of PRBC and FFP, leading to a decreased number of patients who could potentially develop blood transfusions–related complications. Although the effect of TEG/ROTEM was inconsistent on other selected outcomes (the need for additional hemostatic interventions and mortality), the benefit from reduced exposure to blood products transfusions, combined with no harm to the patient from using the technology, led us to make this conditional recommendation.
Using these Guidelines in Clinical Practice
This Practice Management Guidelines addresses the role of TEG/ROTEM in guiding transfusions in patients with ongoing hemorrhage and concern for coagulopathy in adult trauma patients, surgical patients, and critically ill patients. After performing a formal, exhaustive literature search and assessing the existing evidence with the GRADE methodology, we conditionally recommend using TEG/ROTEM-guided blood product transfusions (Table 2). The TEG/ROTEM-guided transfusions led to fewer numbers of patients exposed to blood product transfusions in all studied populations and to fewer blood product transfusions per patient in trauma and surgical patients. Recognizing differences in resources between institutions in treating such patients, we recommend incorporating TEG/ROTEM into local institutional protocols. These recommendations should complement, not replace, clinical judgment.
This systematic review has few limitations that among others included a risk for incomplete retrieval of identified research, selection bias for the procedure, and publication bias due to the mainly positive published results. In studies where number of transfused blood products was reported, it was unclear what denominator was used: either total number of patients in the corresponding study arm or only those who received transfusions. All included studies reported utilization of TEG/ROTEM per the local institutional protocols. Unfortunately, the heterogeneity of the studied patient populations and local protocols precluded us on making any statements in favor of specific protocols. However, these typically included patients with clinically significant active bleeding and suspected or confirmed coagulopathy, and revolve around repeating the test after guided component transfusion, until TEG/ROTEM normalization and clinical evidence of bleeding cessation. Given the heterogeneity of the population in the included studies, adjustments based on age, mechanism of injury/associated diagnoses, comorbidities, injury severity, and physiologic derangement were unable to be performed. Lack of cost data did not allow us to conduct a cost analysis into our recommendations.
Conclusions
We conditionally recommend using TEG/ROTEM to guide blood transfusions instead of traditional coagulation parameters in each of the following three groups: adult trauma patients, adult surgical patients, and adult critically ill patients with ongoing hemorrhage and concern for coagulopathy.
Authorship
N.B. contributed in the study design, literature search, data collection, data analysis, data interpretation, and drafting of the article. J.J.C., G.G., J.J.F., J.S.S., C.J.V., B.K.Y., L.A.K., N.M.G., H.A.A., P.A.P., E.J.M., and Z.W.B. contributed in the study design, data collection, data interpretation, and critical revisions. G.K. contributed in the study idea, study design, literature search, data collection, data analysis, data interpretation, and critical revisions.
Acknowledgments
We thank Judy Rabinowitz, a medical librarian from the Hirsh Health Sciences Library, for the meticulous literature search.
Disclosure
The authors declare no conflicts of interest.
References
- Dobson GP, Morris JL, Davenport LM, Letson HL. Traumatic-induced coagulopathy as a systems failure: a new window into hemostasis. Semin Thromb Hemost. 2020;46(2):199–214.
- Segal JB, Dzik WH. Paucity of studies to support that abnormal coagulation test results predict bleeding in the setting of invasive procedures: an evidence-based review. Transfusion. 2005;45:1413–1425. View Full Text | PubMed | CrossRef
- Dzik WH. Predicting hemorrhage using preoperative coagulation screening assays. Curr Hematol Rep. 2004;3(5):324–330. PubMed
- Tripodi A, Caldwell SH, Hoffman M, Trotter JF, Sanyal AJ. Review article: the prothrombin time test as a measure of bleeding risk and prognosis in liver disease. Aliment Pharmacol Ther. 2007;26(2):141–148. View Full Text | PubMed | CrossRef
- Lim HY, O’Malley C, Donnan G, Nandurkar H, Ho P. A review of global coagulation assays — is there a role in thrombosis risk prediction?Thromb Res. 2019;179:45–55. PubMed | CrossRef
- Hartmann J, Walsh M, Grisoli A, et al. Diagnosis and treatment of trauma-induced coagulopathy by Viscoelastography. Semin Thromb Hemost. 2020;46(2):134–146.
- Benes J, Zatloukal J, Kletecka J. Viscoelastic methods of blood clotting assessment: multidisciplinary review. Front Med (Lausanne). 2015;2:62.
- Kwaan HC. The central role of fibrinolytic response in trauma-induced coagulopathy: a hematologist’s perspective. Semin Thromb Hemost. 2020;46(2):116–124.
- Longstaff C. Measuring fibrinolysis: from research to routine diagnostic assays. J Thromb Haemost. 2018;16(4):652–662. View Full Text | PubMed | CrossRef
- Wikkelsø A, Wetterslev J, Møller AM, Afshari A. Thromboelastography (TEG) or thromboelastometry (ROTEM) to monitor haemostatic treatment versus usual care in adults or children with bleeding. Cochrane Database Syst Rev. 2016;2016(8):CD007871. PubMed
- Kerwin AJ, Haut ER, Burns JB, Como JJ, Haider A, Stassen N, Dahm P; Eastern Association for the Surgery of Trauma Practice Management Guidelines Ad Hoc Committee. The Eastern Association of the Surgery of Trauma approach to practice management guideline development using Grading of Recommendations, Assessment, Development, and Evaluation (GRADE) methodology. J Trauma Acute Care Surg. 2012;73(5 Suppl 4):S283–S287.
- Gonzalez E, Moore EE, Moore HB, et al. Goal-directed hemostatic resuscitation of trauma-induced coagulopathy: a pragmatic randomized clinical trial comparing a viscoelastic assay to conventional coagulation assays. Ann Surg. 2016;263(6):1051–1059. View Full Text | PubMed | CrossRef
- Guth C, Vassal O, Friggeri A, Wey PF, Inaba K, Decullier E, Ageron FX, David JS. Effects of modification of trauma bleeding management: a before and after study. Anaesth Crit Care Pain Med. 2019;38(5):469–476.
- Nardi G, Agostini V, Rondinelli B, Russo E, Bastianini B, Bini G, Bulgarelli S, Cingolani E, Donato A, Gambale G, Ranaldi G. Trauma-induced coagulopathy: impact of the early coagulation support protocol on blood product consumption, mortality and costs. Crit Care. 2015;19(1):83. PubMed | CrossRef
- Prat NJ, Meyer AD, Ingalls NK, Trichereau J, DuBose JJ, Cap AP. Rotational thromboelastometry significantly optimizes transfusion practices for damage control resuscitation in combat casualties. J Trauma Acute Care Surg. 2017;83(3):373–380.
- Schaden E, Kimberger O, Kraincuk P, Baron DM, Metnitz PG, Kozek-Langenecker S. Perioperative treatment algorithm for bleeding burn patients reduces allogeneic blood product requirements. Br J Anaesth. 2012;109(3):376–381. View Full Text | PubMed | CrossRef
- Schöchl H, Nienaber U, Maegele M, Hochleitner G, Primavesi F, Steitz B, Arndt C, Hanke A, Voelckel W, Solomon C. Transfusion in trauma: thromboelastometry-guided coagulation factor concentrate-based therapy versus standard fresh frozen plasma-based therapy. Crit Care. 2011;15(2):R83. PubMed | CrossRef
- Unruh M, Reyes J, Helmer SD, Haan JM. An evaluation of blood product utilization rates with massive transfusion protocol: before and after thromboelastography (TEG) use in trauma. Am J Surg. 2019;218(6):1175–1180. PubMed | CrossRef
- Ak K, Isbir CS, Tetik S, Atalan N, Tekeli A, Aljodi M, Civelek A, Arsan S. Thromboelastography-based transfusion algorithm reduces blood product use after elective CABG: a prospective randomized study. J Card Surg. 2009;24(4):404–410. View Full Text | PubMed | CrossRef
- Bakhshandeh H, Alavi M, Ghadrdoost B, Farsad F, Zavarei A, Zareifard M, Farasatkish R, Babaei T, Baharestani B, Golpira R. Role of ROTEM in hemostatic management during adult cardiac surgery. Res Cardiovasc Med. 2016;5(4):7. Available at: https://www.rcvmonline.com/temp/ResCardiovascMed547-4331579_120155.pdf.
- Girdauskas E, Kempfert J, Kuntze T, Borger MA, Enders J, Fassl J, Falk V, Mohr FW. Thromboelastometrically guided transfusion protocol during aortic surgery with circulatory arrest: a prospective, randomized trial. J Thorac Cardiovasc Surg. 2010;140(5):1117–1124. View Full Text | PubMed | CrossRef
- Görlinger K, Dirkmann D, Hanke AA, Kamler M, Kottenberg E, Thielmann M, Jakob H, Peters J. First-line therapy with coagulation factor concentrates combined with point-of-care coagulation testing is associated with decreased allogeneic blood transfusion in cardiovascular surgery: a retrospective, single-center cohort study. Anesthesiology. 2011;115(6):1179–1191. View Full Text | PubMed | CrossRef
- Ichikawa J, Marubuchi T, Nishiyama K, Kodaka M, Görlinger K, Ozaki M, Komori M. Introduction of thromboelastometry-guided administration of fresh-frozen plasma is associated with decreased allogeneic blood transfusions and post-operative blood loss in cardiopulmonary-bypass surgery. Blood Transfus. 2018;16(3):244–252.
- Manikappa S, Mehta Y, Juneja R, Trehan N. Changes in transfusion therapy guided by thromboelastograph in cardiac surgery. Ann Card Anaesth. 2001;4(1):21–27. PubMed
- Redfern RE, Fleming K, March RL, Bobulski N, Kuehne M, Chen JT, Moront M. Thrombelastography-directed transfusion in cardiac surgery: impact on postoperative outcomes. Ann Thorac Surg. 2019;107(5):1313–1318.
- Royston D, von Kier S. Reduced haemostatic factor transfusion using heparinase-modified thrombelastography during cardiopulmonary bypass. Br J Anaesth. 2001;86(4):575–578. View Full Text | PubMed | CrossRef
- Shi H, Shi B, Lu J, Wu L, Sun G. Application value of thromboelastography in perioperative clinical blood transfusion and its effect on the outcome of patient. Exp Ther Med. 2019;17(5):3483–3488.
- Shore-Lesserson L, Manspeizer HE, DePerio M, Francis S, Vela-Cantos F, Ergin MA. Thromboelastography-guided transfusion algorithm reduces transfusions in complex cardiac surgery. Anesth Analg. 1999;88(2):312–319. View Full Text | PubMed | CrossRef
- Smart L, Mumtaz K, Scharpf D, Gray NO, Traetow D, Black S, Michaels AJ, Elkhammas E, Kirkpatrick R, Hanje AJ. Rotational thromboelastometry or conventional coagulation tests in liver transplantation: comparing blood loss, transfusions, and cost. Ann Hepatol. 2017;16(6):916–923. PubMed | CrossRef
- Smith I, Pearse BL, Faulke DJ, Naidoo R, Nicotra L, Hopkins P, Ryan EG. Targeted bleeding management reduces the requirements for blood component therapy in lung transplant recipients. J Cardiothorac Vasc Anesth. 2017;31(2):426–433. PubMed | CrossRef
- Spiess BD, Gillies BS, Chandler W, Verrier E. Changes in transfusion therapy and reexploration rate after institution of a blood management program in cardiac surgical patients. J Cardiothorac Vasc Anesth. 1995;9(2):168–173. PubMed | CrossRef
- Trzebicki J, Flakiewicz E, Kosieradzki M, et al. The use of thromboelastometry in the assessment of hemostasis during orthotopic liver transplantation reduces the demand for blood products. Ann Transplant. 2010;15(3):19–24. PubMed
- Vasques F, Spiezia L, Manfrini A, Tarzia V, Fichera D, Simioni P, Gerosa G, Ori C, Di Gregorio G. Thromboelastometry guided fibrinogen replacement therapy in cardiac surgery: a retrospective observational study. J Anesth. 2017;31(2):286–290.
- Wang SC, Shieh JF, Chang KY, Chu YC, Liu CS, Loong CC, Chan KH, Mandell S, Tsou MY. Thromboelastography-guided transfusion decreases intraoperative blood transfusion during orthotopic liver transplantation: randomized clinical trial. Transplant Proc. 2010;42(7):2590–2593. PubMed | CrossRef
- Weber CF, Görlinger K, Meininger D, Herrmann E, Bingold T, Moritz A, Cohn LH, Zacharowski K. Point-of-care testing: a prospective, randomized clinical trial of efficacy in coagulopathic cardiac surgery patients. Anesthesiology. 2012;117(3):531–547. View Full Text | PubMed | CrossRef
- Westbrook AJ, Olsen J, Bailey M, Bates J, Scully M, Salamonsen RF. Protocol based on thromboelastograph (TEG) out-performs physician preference using laboratory coagulation tests to guide blood replacement during and after cardiac surgery: a pilot study. Heart Lung Circ. 2009;18(4):277–288.
- Yildirim F, Tuncer B, Ozbakkaloglu A, Kurdal AT, Ozturk T, Iskesen I. Thromboelastogram reduces blood use by inspecting coagulation in heart surgery. Asian Cardiovasc Thorac Ann. 2016;24(5):441–444.
- Monaco F, Barucco G, Nardelli P, Licheri M, Notte C, De Luca M, Mattioli C, Melissano G, Chiesa R, Zangrillo A. Editor’s choice — a rotational thromboelastometry driven transfusion strategy reduces allogenic blood transfusion during open thoraco-abdominal aortic aneurysm repair: a propensity score matched study. Eur J Vasc Endovasc Surg. 2019;58(1):13–22.
- Nascimento JCR, Neto EBL, da Silva EL, et al. Analysis of the hemostatic therapy in liver transplantation guided by rotational thromboelastometry or conventional laboratory tests [published online February 27, 2020]. Eur J Gastroenterol Hepatol. doi:10.1097/MEG.0000000000001660.
- Anderson L, Quasim I, Soutar R, Steven M, Macfie A, Korte W. An audit of red cell and blood product use after the institution of thromboelastometry in a cardiac intensive care unit. Transfus Med. 2006;16(1):31–39. View Full Text | PubMed | CrossRef
- Aoki K, Sugimoto A, Nagasawa A, Saito M, Ohzeki H. Optimization of thromboelastography-guided platelet transfusion in cardiovascular surgery. Gen Thorac Cardiovasc Surg. 2012;60(7):411–416. PubMed
- Avidan MS, Alcock EL, Da Fonseca J, Ponte J, Desai JB, Despotis GJ, Hunt BJ. Comparison of structured use of routine laboratory tests or near-patient assessment with clinical judgement in the management of bleeding after cardiac surgery. Br J Anaesth. 2004;92(2):178–186. View Full Text | PubMed | CrossRef
- Deaton BR, Villalobos NE, Mytinger AK, Youngs GK, Vazquez Guillamet R. ROTEM in the management of upper gastrointestinal bleeding in the intensive care unit. Am J Respir Crit Care Med. 2017;195:A5786. Available at: https://www.atsjournals.org/doi/pdf/10.1164/ajrccm-conference.2017.195.1_MeetingAbstracts.A5786.
- Kumar M, Ahmad J, Maiwall R, et al. Thromboelastography-guided blood component use in patients with cirrhosis with nonvariceal bleeding: a randomized controlled trial. Hepatology. 2020;71(1):235–246. View Full Text | PubMed | CrossRef
- McNamara H, Kenyon C, Smith R, Mallaiah S, Barclay P. Four years’ experience of a ROTEM® -guided algorithm for treatment of coagulopathy in obstetric haemorrhage. Anaesthesia. 2019;74(8):984–991. View Full Text | PubMed | CrossRef
- Saeveraas SB, Seghatchian J, Sivertsen J, Hervig T. The use of thromboelastography (TEG) in massively bleeding patients at Haukeland University Hospital 2008-15. Transfus Apher Sci. 2019;58(1):117–121. PubMed
- Snegovskikh D, Souza D, Walton Z, Dai F, Rachler R, Garay A, Snegovskikh VV, Braveman FR, Norwitz ER. Point-of-care viscoelastic testing improves the outcome of pregnancies complicated by severe postpartum hemorrhage. J Clin Anesth. 2018;44:50–56.
- Vymazal T, Astraverkhava M, Durila M. Rotational thromboelastometry helps to reduce blood product consumption in critically ill patients during small surgical procedures at the intensive care unit — a retrospective clinical analysis and literature search. Transfus Med Hemother. 2018;45(6):385–387.
- Rout G, Shalimar, Gunjan D, Mahapatra SJ, Kedia S, Garg PK, Nayak B. Thromboelastography-guided blood product transfusion in cirrhosis patients with variceal bleeding: a randomized controlled trial. J Clin Gastroenterol. 2020;54(3):255–262. Gastroenterol Hepatol.
- Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA, eds. Cochrane Handbook for Systematic Reviews of Interventions. 2nd ed. Chichester, United Kingdom: John Wiley & Sons; 2019.
- The Cochrane Collaboration. Cochrane Community. Available at: https://community.cochrane.org/help/tools-and-software/gradepro-gdt. Accessed July 20, 2020.
Keywords:
Thromboelastography; TEG; rotational thromboelastometry; ROTEM; hemorrhage

