Whole Blood Resuscitation for Injured Patients Requiring Transfusion: A Systematic Review, Meta-Analysis, and Practice Management Guideline from the Eastern Association for the Surgery of Trauma
Published 2024
Citation: Journal of Trauma and Acute Care Surgery 97(3):p 460-470, September 2024. | DOI: 10.1097/TA.0000000000004327
Authors
Jonathan P. Meizoso, MD, MSPH; Bryan A. Cotton, MD, MPH; Ryan A. Lawless, MD; Lisa M. Kodadek, MD; Jennifer M. Lynde, DO; Nicole Russell; John Gaspich, MD; Adrian Maung, MD; Christofer Anderson, MD; John M. Reynolds, MLIS; Krista L. Haines, DO; George Kasotakis, MD, MPH; and Jennifer J. Freeman, MD, MPH
Introduction
Uncontrolled hemorrhage is the leading cause of death among injured civilian and combat casualties [1][2]. The adoption of damage control resuscitation, which includes the principles of limited crystalloid use, incorporation of massive transfusion protocols, and permissive hypotension, has resulted in significant reductions in mortality after trauma [3][4]. Despite this, hemorrhagic shock currently accounts for approximately one-third of all traumarelated
mortality [1][5].
Balanced blood product resuscitation with equal or near equal ratios of packed red blood cells (RBC), plasma, and platelets (i.e., 1:1:1 RBC to plasma to platelets) is the cornerstone of current transfusion practices for injured patients [6]. Prior data have demonstrated that ratios of RBC, plasma, and platelets approaching 1:1:1 are associated with improved outcomes [7], presumably secondary to achieving ratios of individual blood components similar to the
composition of shed whole blood (WB). While balanced component therapy (COMP) is unquestionably superior to crystalloid resuscitation, coagulopathy and decreased hemostatic potential are still evident when individual components are transfused [8-10].
Resuscitation with WB is a practice that dates back to at least the 18th century and was the standard of care for combat casualties through the Vietnam War [3][11]. This practice shifted to a component-based approach with the advent of blood fractionation techniques, which allowed 1 unit of WB to be split into multiple products to treat multiple patients; of note, there were no data at the time to evaluate whether component-based therapy was equivalent to a WB approach
[3]. Recently, there has been renewed interest in employing WB-based resuscitation for trauma patients in both the military and civilian settings. Potential benefits of WB include improved hemostatic capacity and decreased overall volume requirements when transfusing WB compared to COMP. However, the effect of a WB-based resuscitation strategy on mortality for injured patients remains unclear.
An Eastern Association for the Surgery of Trauma (EAST) work group was formed to conduct a systematic review and meta-analysis. The primary objective was to develop evidencebased recommendations on whether WB should be considered in civilian trauma patients receiving blood transfusions, following the Grading of Recommendations Assessment, Development and Evaluation (GRADE) methodology [12-14].
Objectives
The primary objective of this guideline was to assess whether resuscitation with WB should be considered in the management of civilian trauma patients requiring blood transfusion compared to resuscitation with COMP alone. An EAST work group conducted a formal systematic review and meta-analysis for quantitative evaluation, and used these data as the basis for our recommendations in strict accordance with GRADE methodology [12-14].
Per the GRADE methodology, an a priori question following the Population (P), Intervention (I), Comparison (C), and Outcomes (O) (PICO) format was developed. We identified one PICO stem by consensus of the work group. Several potential candidate outcomes were considered, and voting took place independently on a scale from 1 to 9 by each author per the GRADE methodology. Critical outcome variables received a score of 7 – 9 and comprised the outcomes included in our study. Outcome variables scoring 4 – 6 (important) and 1 – 3 (limited importance) were not included in the analysis. The list of potential outcomes and scoring results are presented in the Supplemental Digital Content Table 1, http://links.lww.com/TA/D686.
The final PICO question was developed a priori, before the systematic literature search:
In adult civilian trauma patients receiving blood transfusions (P), should resuscitation with whole blood (I) be used versus resuscitation with component therapy alone (C) to decrease mortality (O1), transfusion requirements (O2), infectious complications (O3), or intensive care unit (ICU) length of stay (LOS) (O4)?
Methods
This systematic review was registered on the International Prospective Register of Systematic Reviews (PROSPERO, https://www.crd.york.ac.uk/prospero/), Registration No. #CRD42023451143, and was conducted with guidance from the Methodological Expectations of Cochrane Intervention Reviews Manual (MECIR, https://community.cochrane.org/mecirmanual) and reported in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA, https://www.prisma-statement.org). The PRISMA 2020 Checklist and PRISMA 2020 for Abstracts Checklist are available as Supplemental Digital Content Table 2 and Table 3, http://links.lww.com/TA/D686.
Search strategy
An electronic database search strategy was developed by an academic health science librarian (J.M.R.) in consultation with the primary authors (J.P.M. and J.J.F.) and was reviewed by another health science librarian using the Peer Review for Electronic Search Strategies tool [15]. A combination of controlled-vocabulary subject headings and text words were used for the concepts of WB, component blood, transfusions, traumatic injuries, resuscitation, and their synonyms. The search was conducted in the Medline (Ovid), Embase (Elsevier), Cochrane CENTRAL (Wiley), and CINAHL Plus with Full Text (Ebsco) databases, and the Web of Science platform (Clarivate). The search strategy was written for Medline and translated using each database's syntax, subject headings, and search fields. The search strategy was adapted for other databases in part with the use of the Institute for Evidence Based Healthcare's Polyglot Search translator [16]. Results were limited to human studies by excluding animal research. No language, date, or other limits were applied at the search phase. No trial registries, tables of contents, or other resources were searched. No study authors were contacted for additional data. All databases were searched on May 30, 2023. Citations from related systematic reviews were screened with database records. For complete search strategies and details of databases and segments used as well as selected systematic reviews used in citation searching, see Supplemental Digital Content Table 4, http://links.lww.com/TA/D686. All database records were downloaded to EndNote X9 (The EndNote Team, 2021) and uploaded to Covidence webbased software (Veritas Health Innovation, https://www.covidence.org, 2023) for deduplication, screening, and full-text evaluation. The Retraction Watch database was checked via EndNote software for retractions of included studies.
Study selection
Inclusion criteria were English language studies conducted in adult civilian trauma patients that compared WB to COMP during their initial resuscitation phase in the hospital. Studies that primarily focused on patients receiving prehospital transfusions and those conducted in military or pediatric populations were excluded. Of note, due to the variable age cutoffs for pediatric trauma patients across centers, 6 studies included patients < 18 years of age, with the youngest included patients being 15 years of age. Additionally, we excluded animal studies, case reports, and case series.
Data extraction and management
Upon completion of the literature search, titles, abstracts, and full-text manuscripts were reviewed by two independent reviewers on the Task Force. Non-English language studies were excluded at this point. Conflicts between two reviewers were independently adjudicated by a third reviewer. Data from each manuscript selected for inclusion were then extracted independently by two reviewers using a standardized data extraction sheet in Microsoft Excel (Microsoft Corporation, Redmond, WA).
Statistical analysis
Meta-analyses were performed using RevMan (The Cochrane Collaboration, https://revman.cochrane.org). Odds ratios and 95% confidence intervals for binary outcomes and differences in means with 95% confidence intervals for continuous outcomes were calculated. Statistical significance was set at p < 0.05. I2 was calculated to determine heterogeneity, with I2 values less than 50% indicating a low degree of heterogeneity, I2 values between 50% and 74% indicating moderate heterogeneity, and I2 values greater than 75% indicating high heterogeneity[17].
Assessment of the quality of evidence
GRADEpro (McMaster University; Evidence Prime, Inc.; https://www.gradepro.org) was employed to assess the quality of evidence. Quality assessment considered the potential risk of bias, inconsistency, indirectness, imprecision, and publication bias. The quality of the body of evidence was rated as high, moderate, low, or very low, consistent with the GRADE approach.
Creation of recommendations
The results of the meta-analyses and assessment of the quality of evidence were used to inform subsequent recommendations. Every member of the work group independently voted on recommendations for the PICO question and all associated outcomes, taking into consideration the potential risks and benefits of treatment, patient values and preferences, and resource utilization, in addition to the objective metrics provided by the meta-analyses and quality assessment. Each step in developing the following practice management guidelines strictly followed GRADE methodology [12-14].
Results
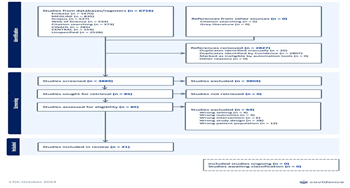
The literature search identified 6,716 studies. Twenty-one studies met inclusion criteria and were included in the final analysis [18-38] (see PRISMA flow diagram, Figure 1). A summary table of included studies, baseline characteristics, and outcomes is presented in Supplemental Digital Content Table 5, http://links.lww.com/TA/D686.
PICO: In adult civilian trauma patients receiving blood transfusions, should resuscitation with whole blood be used versus resuscitation with component therapy alone to decrease mortality, transfusion requirements, infectious complications, or ICU LOS?
Qualitative analysis
Twenty-one studies were included in the analysis, with publication dates from 2013 to 2023. The earliest study by Cotton et al. was the only randomized controlled trial included [20]. In this small pilot trial, 107 patients meeting highest-level trauma activation criteria with evidence of bleeding and need for emergency release of blood products in the emergency department (ED) were randomized to receive modified WB (n = 55) or COMP (n = 52). Median transfusions at 24 hours, 24-hour mortality, and 30-day mortality were not different between groups on univariate analysis. However, sensitivity analysis excluding patients with severe traumatic brain injury demonstrated a decrease in 24-hour transfusion requirements among patients receiving modified WB [20].
Three large prospective observational studies, including two multicenter investigations, have demonstrated benefit of WB compared to COMP. In a single center study of 1,377 patients receiving emergency release blood by Brill et al., WB was associated with higher adjusted 30-day survival and reduced 24-hour blood product use (19). Hazelton et al. reported lower 4-hour and 24-hour RBC transfusions on univariate analyses as well as decreased mortality and bleeding complications on multivariate analyses in patients receiving WB compared to COMP, with no differences in major complications [25]. A recent prospective multicenter observational study of injured patients at risk of massive transfusion who required both blood transfusion and hemorrhage control procedures found no significant differences in 4-hour, 24-hour, or 28-day mortality between patients receiving WB or COMP on univariate or multivariate analyses; however, when adjusting for patients arriving with elevated probability of mortality, WB was associated with lower risk of 4-hour, 24-hour, and 28-day mortality compared to COMP [35].
In the 18 studies that reported mortality outcomes, on univariate analysis, 12 studies (67%) demonstrated no difference between WB or COMP on any mortality outcome (i.e., early, 24-hour, 28- or 30-day, or overall mortality) [18-22][24][27][28][31][35][37][38], five studies (28%) demonstrated decreased mortality at some time point in patients receiving WB compared to COMP [21][23][24][29][36], and one study (6%) suggested that WB was associated with higher overall mortality compared to COMP [26]. Seven studies performed multivariate analyses, including 2 studies (29%) that reported no difference between WB and COMP [29][35] and five studies (71%) that reported a mortality benefit in patients receiving WB compared to COMP [19][25-27][33]. No studies reported elevated mortality in the WB group on multivariate analysis.
Seventeen studies reported transfusion outcomes [18-25][28-34][37][38]. Of these, 11 studies (65%) demonstrated a reduction in transfusions in patients receiving WB compared to COMP [18][21][22][25][30-34][37][38], four studies (24%) demonstrated no difference between WB and COMP [20][23][24][29], and only one study (6%) demonstrated more transfusions in patients receiving WB compared to COMP in at least one time point on univariate analysis [28]. Two studies reporting results of multivariate analyses demonstrated decreased 24-hour overall transfusions [19] and higher plasma to RBC ratios [34] in patients who received WB compared to COMP.
Ten studies demonstrated no significant differences in ICU LOS or ICU-free days between patients receiving WB or COMP. No studies reported differences in infectious complications between groups.
Overall, the included studies enrolled similar patient populations, including patients in hemorrhagic shock, at risk of massive transfusion, and receiving emergency-release blood. Additionally, outcomes definitions were similar among the studies, with the majority using established standards for hemorrhage-related studies [39]. Despite this, there was considerable variation in the results, particularly for mortality-related outcomes, which may be a consequence of differences in injury severity and massive transfusion protocols between institutions, and inclusion of certain high-risk populations (e.g., severe traumatic brain injury). However, when these differences were adjusted for using multivariate techniques, most studies demonstrated a mortality benefit in patients who received WB. Additionally, WB demonstrated a consistent benefit over COMP in terms of decreased blood transfusion and higher ratios of plasma and platelets to RBC in a majority of studies, without significant differences in infectious complications or length of stay. Finally, it should be noted that the type of WB used was different among studies. Eight studies reported use of low-titer group O WB leukoreduced with a platelet-sparing filter [18][22][24][31][33][34][37][38], 3 studies reported the use leukoreduced low-titer group O WB without mention of a platelet-sparing filter [25][29][30], 1 study used lowtiter group O WB without mention of leukoreduction [28], 1 study specifically mentioned that the low-titer group O WB was not leukoreduced [19], 1 study used modified WB with nonfunctional platelets [20], and 7 studies did not specify the type of WB [21][23][26][27][32][35][36]. This variation in the type of product used may have an impact on our study outcomes.
Quantitative analysis
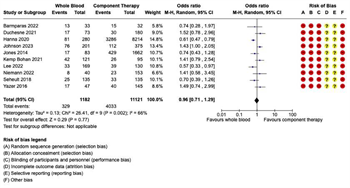
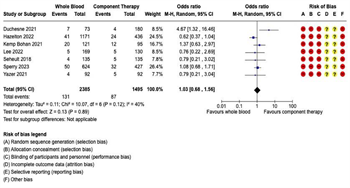
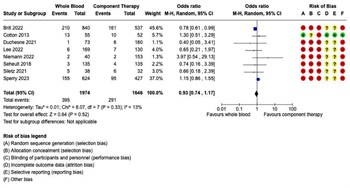
Figure 2: Forest Plots for Mortality. (A) Early mortality, including emergency department, 3- hour, and 6-hour mortality, where available. (B) 24-hour mortality. (C) Late mortality, including 28-day or 30-day mortality, where available. (D) In-hospital mortality.
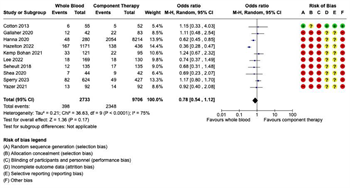
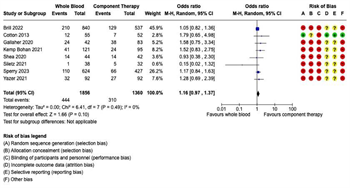
Mortality: Eighteen studies reported mortality outcomes with sufficient data for inclusion in the meta-analysis. Given the variation in the mortality time points reported, separate meta-analyses were performed for each of the following four mortality outcomes: (1) early mortality, defined as ED, 3-hour, or 6-hour mortality; (2) 24-hour mortality; (3) late mortality, defined as 28-day or 30-day mortality; and (4) overall in-hospital mortality (Figure 2). In the seven studies reporting early mortality, 2,385 patients received WB and 1,495 received COMP. There were 131 deaths in the WB group and 87 deaths in the COMP group. Pooled data demonstrated no significant difference on early mortality between the WB and COMP groups (OR 1.03, 95% CI 0.68 – 1.56) and heterogeneity was low (I2 = 40%) (Figure 2A). Ten studies reported 24-hour mortality, with 2,733 patients receiving WB and 9,706 receiving COMP. There were 398 deaths in the WB group and 2,348 in the COMP group. Pooled data demonstrated no significant difference in 24-hour mortality between WB and COMP (OR 0.78, 95% CI 0.54 – 1.12). Heterogeneity was high (I2 = 75%) (Figure 2B). Eight studies reported late mortality. There were 1,856 patients who received WB and 1,360 who received COMP, with 444 deaths in the WB group and 310 in the COMP group. There were no significant differences in late mortality between WB and COMP (OR 1.16, 95% CI .97 – 1.37) and heterogeneity was low (I2 = 0%) (Figure 2C). Finally, 10 studies reported overall in-hospital mortality, with 1,182 patients in the WB group and 11,121 in the COMP group. There were 329 deaths in the WB group and 4,033 in the COMP group. Pooled data demonstrated no significant differences in overall in-hospital mortality between groups (OR 0.96, 95% CI 0.71 – 1.29), with moderate heterogeneity among studies (I2 = 66%) (Figure 2D).
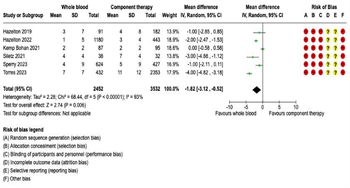
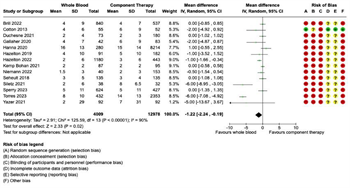
Transfusion Requirements: Fourteen studies were included in the meta-analysis for transfusion requirements. Separate meta-analyses were performed for each of the following five transfusion outcomes: (1) 4-hour RBC transfusions; (2) 4-hour plasma transfusions; (3) 24-hour RBC transfusions; (4) 24-hour plasma transfusions; and (5) 24-hour total transfusions (Figure 3 and Figure 4). Six studies reported 4-hour RBC and plasma transfusion requirements. There were 2,452 patients in the WB group and 3,532 in the COMP group. Pooled analysis demonstrated decreased 4-hour RBC transfusions (mean difference -1.82, 95% CI -3.12 to -0.52, I2 = 93%) and decreased 4-hour plasma transfusions (mean difference -1.47, 95% CI -2.94 to 0, I2 = 95%) in patients receiving WB compared to COMP (Figure 3). Fourteen studies reported 24-hour RBC and plasma transfusions, with 4,009 patients in the WB group and 12,978 in the COMP group.
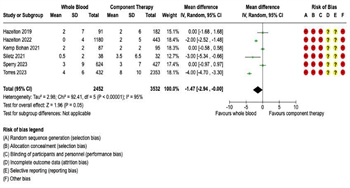
Figure 3: Forest Plots for 4-Hour Transfusion Requirements. (A) Red blood cell transfusion requirements. (B) Plasma transfusion requirements.
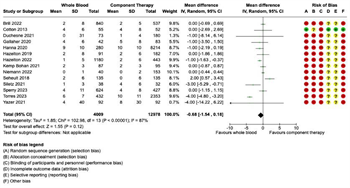
Pooled data demonstrated decreased 24-hour RBC transfusions in patients receiving WB compared to COMP (mean difference -1.22, 95% CI -2.24 to -0.19, I2 = 90%) (Figure 4A), but no significant difference in 24-hour plasma transfusions (mean difference -0.68, 95% CI -1.54 to 0.18, I2 = 87%) (Figure 4B). Seven studies reported 24-hour total transfusions. There were 3,013 patients in the WB group and 1,781 patients in the COMP group. No significant differences were noted between WB and COMP on 24-hour total transfusions (mean difference 0.56, 95% CI - 1.57 to 2.69, I2 = 78%) (Figure 4C).
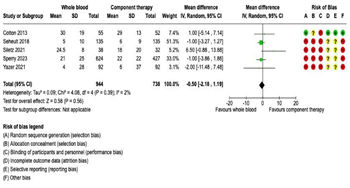
Intensive Care Unit Length of Stay: Twelve studies reported ICU LOS outcomes. Of these, five reported ICU-free days and nine reported ICU days. Therefore, separate meta-analyses were performed for (1) ICU-free days and (2) ICU days. There were no significant differences in ICUfree days on pooled analysis between patients receiving WB (n = 944) or COMP (n = 738) (mean difference -0.50, 95% CI -2.18 to 1.19, I2 = 2%) (Figure 5A). Similarly, there were no significant differences in ICU days on pooled analysis between patients receiving WB (n = 1,117) or COMP (n = 3,366) (mean difference -0.35, 95% CI -1.34 to 0.63, I2 = 46%) (Figure 5B).
Infectious Complications: Eight studies reported on infections complications. There were 1,974 patients in the WB group and 1,646 in the COMP group, with 395 infectious complications in the WB group and 291 in the COMP group. Pooled analysis demonstrated no significant difference in infectious complications between WB and COMP (OR 0.93, 95% CI 0.74 – 1.17). Heterogeneity was low (I2 = 13%) (Figure 6).
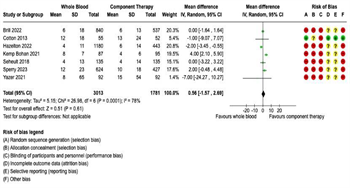
Figure 4: Forest Plots for 24-Hour Transfusion Requirements. (A) Red blood cell transfusion requirements. (B) Plasma transfusion requirements. (C) Total transfusion requirements.
Grading the evidence
Of the 21 studies included, 13 were retrospective [18][22-24][26-29][31][32][36-38], with three studies utilizing large national databases during different time periods [23][27][36], seven were prospective observational studies [19][21][25][30][33-35], of which two were large, multicenter studies [25][35], and only one was a randomized controlled trial [20]. All studies included patients who either presented in shock, required blood transfusion in the ED, were predicted to or required a massive transfusion, and/or required a hemorrhage control procedure. The risk of bias was considered serious to very serious due to the large proportion of retrospective studies. Similarly, inconsistency was considered a problem for the majority of studies due to the variation in reported outcomes. Overall, imprecision was considered serious for most outcomes, except the mortality outcomes, where the confidence intervals were noted to be narrow. Publication bias was not deemed to be a concern given several negative studies were published for various outcomes. Therefore, the overall quality of evidence was deemed to be very low (Supplemental Digital Content Table 6, http://links.lww.com/TA/D686). The Risk of Bias graph and Risk of Bias Summary are depicted in Supplemental Digital Content Figure 1 and Figure 2, http://links.lww.com/TA/D687.
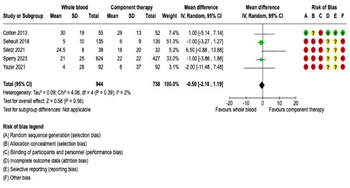
Figure 5: Forest Plots for Intensive Care Unit Length of Stay. (A) Intensive care unit-free days. (B) Intensive care unit days.
Limitations
This systematic review has several important limitations to consider when interpreting the results. First, most of the included studies were retrospective, limiting the ability to establish anything more than an association between WB transfusion and outcomes. Second, the nature of the meta-analysis requires that only raw numbers of events occurring in each group be entered into the analysis. This methodology is likely inconsequential when the majority of studies included are randomized controlled trials, where the populations in each group should be similar with the exception of the intervention. However, for retrospective and prospective observational studies, where there are significant differences in the populations at baseline, the meta-analysis does not take these differences into account. For this systematic review, several studies demonstrated a significant benefit of WB over COMP after making appropriate multivariate adjustments that were not apparent on univariate analysis. Third, most studies evaluated all patients who presented in shock, required or were expected to require a massive transfusion, received blood in the ED, or required a hemorrhage control procedure as part of their resuscitation, without excluding patients with non-survivable injuries. This likely influenced our results. Fourth, the use of retrospective studies utilizing large national databases, which represented a significant proportion of the included patients on meta-analysis, has recently come into question when evaluating hemorrhage-related trauma mortality due to inherent flaws in identifying patients in hemorrhagic shock [40]. Fifth, we excluded studies of pediatric patients from this analysis, although 7 studies did include patients as young as 15 years of age in their adult cohort, and many studies excluded women of childbearing age or did not specify. Therefore, we cannot make any recommendations regarding WB transfusion in these populations. Sixth, only a select few centers initially adopted WB resuscitation in trauma and published their experience in single-center studies that were included in this systematic review and meta-analysis. Undoubtedly, these centers also reported their data to the large national databases used to perform cross-sectional evaluations of WB use in trauma, which were also included in the present study. Additionally, it is likely that centers who published their singlecenter experiences also contributed the same patients to multicenter observational studies that were included in the present study. Overall, this leads to the concern for duplication of patients across the studies included in our analysis and should be considered when interpreting our final recommendation. Finally, our work group chose to exclude patients receiving prehospital transfusion and military studies from this systematic review to focus on interventions available once the patient arrives at the trauma center in the civilian setting. A recent systematic review and meta-analysis that included both military and civilian studies who received WB or COMP either in the prehospital setting or in the ED demonstrated lower early and 24-hour mortality, but not late mortality, in civilian patients treated with WB compared to COMP [41]. Inclusion of studies evaluating prehospital transfusion would likely have influenced our results in favor of WB transfusion when evaluating mortality outcomes.
Recommendations
In formulating these recommendations, we considered the overall quality of the evidence, balance between benefits and harms, patient values and preferences, resource considerations, and acceptability and feasibility. The overall quality of evidence was very low, limiting our ability to formulate strong recommendations for or against the use of WB. Despite this, the risk to benefit ratio of receiving WB vs. COMP was considered acceptable. The potential risk of fetal harm in women of childbearing age is a particular concern related to WB transfusion and was considered when formulating the recommendations. However, several studies evaluating the preferences of women regarding emergency lifesaving transfusions, even when there is a small potential risk of fetal harm, have reported that most women would accept this risk [42-44]. We recognize that resuscitation with WB is a strategy that is re-emerging and may not be feasible at some centers and this was also considered in our recommendation.
Based on these considerations, 7 authors (64%) voted for a conditional recommendation and 4 authors (36%) voted that they could not recommend for or against. Thus, we conditionally recommend using WB in adult civilian trauma patients receiving blood transfusions, recognizing that data are limited for certain populations, including women of childbearing age, and therefore this guideline may not apply to these populations.
Using These Guidelines in Clinical Practice
The overall quality of evidence and lack of a demonstrated difference in mortality outcomes did not allow us to make a strong recommendation for the utilization of WB in the resuscitation of trauma patients who require transfusion. However, given the benefit of using WB in reducing early and 24-hour transfusion volumes, with no apparent effect on studied complications, and demonstration of a survival benefit in the one randomized trial that was available for analysis, we were able to provide a conditional recommendation for its use in the acute setting. It should be noted that these recommendations apply to the acute resuscitation of hemorrhaging trauma patients. Once the early stage of resuscitation has concluded, a transition to an individualized, goal-directed transfusion strategy aimed at correcting specific deficiencies identified on viscoelastic testing or other coagulation parameters with appropriate blood components should be considered.
The incorporation of WB transfusion for trauma patients into practice will largely depend on local institutional capabilities and resources. Currently, the relative scarcity of WB, particularly in non-trauma centers, will inherently limit its widespread use. However, it should be considered in any bleeding adult male or postmenopausal female patient who presents to a trauma center where WB is immediately available. In centers where WB is not immediately available, balanced transfusion using COMP with high ratios of plasma and platelets to RBCs (i.e., 1:1:1) remains a mainstay of treatment. Ongoing randomized trials, including the Type O Whole Blood and Assessment of Age During Prehospital Resuscitation (TOWAR) Trial (ClinicalTrials.gov Identifier: NCT04684719) and the Trauma Resuscitation with Low-Titer Group O Whole Blood or Products (TROOP) Trial (ClinicalTrials.gov Identifier: NCT05638581), are currently enrolling patients and may provide additional evidence for the use of WB in hemorrhaging patients in the future.
References
- Callcut RA, Kornblith LZ, Conroy AS, Robles AJ, Meizoso JP, Namias N, et al. The why and how our trauma patients die: A prospective Multicenter Western Trauma Association study. J Trauma Acute Care Surg. 2019;86(5):864-70.
- Eastridge BJ, Holcomb JB, Shackelford S. Outcomes of traumatic hemorrhagic shock and the epidemiology of preventable death from injury. Transfusion. 2019;59(S2):1423-8.
- Kalkwarf KJ, Cotton BA. Resuscitation for Hypovolemic Shock. Surg Clin North Am. 2017;97(6):1307-21.
- Holcomb JB, Jenkins D, Rhee P, Johannigman J, Mahoney P, Mehta S, et al. Damage control resuscitation: directly addressing the early coagulopathy of trauma. J Trauma. 2007;62(2):307-10.
- Moore EE, Moore HB, Kornblith LZ, Neal MD, Hoffman M, Mutch NJ, et al. Traumainduced coagulopathy. Nat Rev Dis Primers. 2021;7(1):30.
- Cannon JW, Khan MA, Raja AS, Cohen MJ, Como JJ, Cotton BA, et al. Damage control resuscitation in patients with severe traumatic hemorrhage: A practice management guideline from the Eastern Association for the Surgery of Trauma. J Trauma Acute Care Surg. 2017;82(3):605-17.
- Holcomb JB, Tilley BC, Baraniuk S, Fox EE, Wade CE, Podbielski JM, et al. Transfusion of plasma, platelets, and red blood cells in a 1:1:1 vs a 1:1:2 ratio and mortality in patients with severe trauma: the PROPPR randomized clinical trial. JAMA. 2015;313(5):471-82.
- Chambers LA, Chow SJ, Shaffer LE. Frequency and characteristics of coagulopathy in trauma patients treated with a low- or high-plasma-content massive transfusion protocol. Am J Clin Pathol. 2011;136(3):364-70.
- Khan S, Brohi K, Chana M, Raza I, Stanworth S, Gaarder C, et al. Hemostatic resuscitation is neither hemostatic nor resuscitative in trauma hemorrhage. J Trauma Acute Care Surg. 2014;76(3):561-7; discussion 7-8.
- Spahn DR, Rossaint R. Coagulopathy and blood component transfusion in trauma. Br J Anaesth. 2005;95(2):130-9.
- Association for the Advancement of Blood and Biotherapies. Highlights of Transfusion Medicine History. [Available from: https://www.aabb.org/newsresources/ resources/transfusion-medicine/highlights-of-transfusion-medicine-history]. Accessed 08/03/2023.
- Guyatt GH, Oxman AD, Vist GE, Kunz R, Falck-Ytter Y, Alonso-Coello P, et al. GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ. 2008;336(7650):924-6.
- Jaeschke R, Guyatt GH, Dellinger P, Schunemann H, Levy MM, Kunz R, et al. Use of GRADE grid to reach decisions on clinical practice guidelines when consensus is elusive. BMJ. 2008;337:a744.
- Kerwin AJ, Haut ER, Burns JB, Como JJ, Haider A, Stassen N, et al. The Eastern Association of the Surgery of Trauma approach to practice management guideline development using Grading of Recommendations, Assessment, Development, and Evaluation (GRADE) methodology. J Trauma Acute Care Surg. 2012;73(5 Suppl 4):S283-7.
- McGowan J, Sampson M, Salzwedel DM, Cogo E, Foerster V, Lefebvre C. PRESS Peer Review of Electronic Search Strategies: 2015 Guideline Statement. J Clin Epidemiol. 2016;75:40-6.
- Clark JM, Sanders S, Carter M, Honeyman D, Cleo G, Auld Y, et al. Improving the translation of search strategies using the Polyglot Search Translator: a randomized controlled trial. J Med Libr Assoc. 2020;108(2):195-207.
- Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med. 2002;21(11):1539-58.
- Barmparas G, Huang R, Hayes C, Pepkowitz SH, Abumuhor IA, Thomasian SE, et al. Implementation of a low-titer stored whole blood transfusion program for civilian trauma patients: Early experience and logistical challenges. Injury. 2022;53(5):1576-80.
- Brill JB, Tang B, Hatton G, Mueck KM, McCoy CC, Kao LS, et al. Impact of Incorporating Whole Blood into Hemorrhagic Shock Resuscitation: Analysis of 1,377 Consecutive Trauma Patients Receiving Emergency-Release Uncrossmatched Blood Products. J Am Coll Surg. 2022;234(4):408-18.
- Cotton BA, Podbielski J, Camp E, Welch T, del Junco D, Bai Y, et al. A randomized controlled pilot trial of modified whole blood versus component therapy in severely injured patients requiring large volume transfusions. Ann Surg. 2013;258(4):527-32; discussion 32-3.
- Duchesne J, Smith A, Lawicki S, Hunt J, Houghton A, Taghavi S, et al. Single Institution Trial Comparing Whole Blood vs Balanced Component Therapy: 50 Years Later. J Am Coll Surg. 2021;232(4):433-42.
- Gallaher JR, Dixon A, Cockcroft A, Grey M, Dewey E, Goodman A, et al. Large volume transfusion with whole blood is safe compared with component therapy. J Trauma Acute Care Surg. 2020;89(1):238-45.
- Hanna K, Bible L, Chehab M, Asmar S, Douglas M, Ditillo M, et al. Nationwide analysis of whole blood hemostatic resuscitation in civilian trauma. J Trauma Acute Care Surg. 2020;89(2):329-35.
- Hazelton JP, Cannon JW, Zatorski C, Roman JS, Moore SA, Young AJ, et al. Coldstored whole blood: A better method of trauma resuscitation? J Trauma Acute Care Surg. 2019;87(5):1035-41.
- Hazelton JP, Ssentongo AE, Oh JS, Ssentongo P, Seamon MJ, Byrne JP, et al. Use of Cold-Stored Whole Blood is Associated With Improved Mortality in Hemostatic Resuscitation of Major Bleeding: A Multicenter Study. Ann Surg. 2022;276(4):579-88.
- Johnson T, Mack TJ, Burke R, Damiano N, Heger L, Minner N, et al. Whole Blood Trauma Resuscitation in Community Trauma Centers Confers Survival Benefit Over Component Therapy. Am Surg. 2023;89(7):3148-52.
- Jones AR, Frazier SK. Increased mortality in adult patients with trauma transfused with blood components compared with whole blood. J Trauma Nurs. 2014;21(1):22-9.
- Kemp Bohan PM, McCarthy PM, Wall ME, Adams AM, Chick RC, Forcum JE, et al. Safety and efficacy of low-titer O whole blood resuscitation in a civilian level I trauma center. J Trauma Acute Care Surg. 2021;91(2S Suppl 2):S162-S8.
- Lee JS, Khan AD, Wright FL, McIntyre RC, Jr., Dorlac WC, Cribari C, et al. Whole Blood Versus Conventional Blood Component Massive Transfusion Protocol Therapy in Civilian Trauma Patients. Am Surg. 2022;88(5):880-6.
- Niemann BR, Grabo DJ, Mullens C, Shmookler AD, Lopez S, Lander OM, et al. The Use of Whole Blood in Rural Trauma Leads to Decreased Resource Utilization. Am Surg. 2022:31348221142584.
- Seheult JN, Anto V, Alarcon LH, Sperry JL, Triulzi DJ, Yazer MH. Clinical outcomes among low-titer group O whole blood recipients compared to recipients of conventional components in civilian trauma resuscitation. Transfusion. 2018;58(8):1838-45.
- Bush K, Shea L, San Roman J, Pailloz E, Gaughan J, Porter J, et al. Whole Blood in Trauma Resuscitation: What Is the Real Cost? J Surg Res. 2022;275:155-60.
- Shea SM, Staudt AM, Thomas KA, Schuerer D, Mielke JE, Folkerts D, et al. The use of low-titer group O whole blood is independently associated with improved survival compared to component therapy in adults with severe traumatic hemorrhage. Transfusion. 2020;60 Suppl 3:S2-S9.
- Siletz AE, Blair KJ, Cooper RJ, Nguyen NC, Lewis SJ, Fang A, et al. A pilot study of stored low titer group O whole blood + component therapy versus component therapy only for civilian trauma patients. J Trauma Acute Care Surg. 2021;91(4):655-62.
- Sperry JL, Cotton BA, Luther JF, Cannon JW, Schreiber MA, Moore EE, et al. Whole Blood Resuscitation and Association with Survival in Injured Patients with an Elevated Probability of Mortality. J Am Coll Surg. 2023;237(2):206-19.
- Torres CM, Kent A, Scantling D, Joseph B, Haut ER, Sakran JV. Association of Whole Blood With Survival Among Patients Presenting With Severe Hemorrhage in US and Canadian Adult Civilian Trauma Centers. JAMA Surg. 2023;158(5):532-40.
- Yazer MH, Freeman A, Harrold IM, Anto V, Neal MD, Triulzi DJ, et al. Injured recipients of low-titer group O whole blood have similar clinical outcomes compared to recipients of conventional component therapy: A single-center, retrospective study. Transfusion. 2021;61(6):1710-20.
- Yazer MH, Jackson B, Sperry JL, Alarcon L, Triulzi DJ, Murdock AD. Initial safety and feasibility of cold-stored uncrossmatched whole blood transfusion in civilian trauma patients. J Trauma Acute Care Surg. 2016;81(1):21-6.
- Holcomb JB, Moore EE, Sperry JL, Jansen JO, Schreiber MA, Del Junco DJ, et al. Evidence-Based and Clinically Relevant Outcomes for Hemorrhage Control Trauma Trials. Ann Surg. 2021;273(3):395-401.
- Harfouche MN, Feliciano DV, Kozar RA, DuBose JJ, Scalea TM. A Cautionary Tale: The Use of Propensity Matching to Evaluate Hemorrhage-Related Trauma Mortality in the American College of Surgeons TQIP Database. J Am Coll Surg. 2023;236(6):1208-16.
- van der Horst RA, Rijnhout TWH, Noorman F, Borger van der Burg BLS, van Waes OJF, Verhofstad MHJ, et al. Whole blood transfusion in the treatment of acute hemorrhage, a systematic review and meta-analysis. J Trauma Acute Care Surg. 2023;95(2):256-66.
- Uhlich R, Hu P, Yazer M, Jansen JO, Patrician P, Marques MB, et al. The females have spoken: A patient-centered national survey on the administration of emergent transfusions with the potential for future fetal harm. J Trauma Acute Care Surg. 2023;94(6):791-7.
- Uhlich R, Hu P, Yazer M, Jansen JO, Patrician P, Reynolds L, et al. Perception of risk in massive transfusion as it relates to fetal outcomes: A survey of surgeons and nurses at one American trauma center. Transfusion. 2021;61 Suppl 1:S159-S66.
- Yu G, Siegler J, Hayes J, Yazer MH, Spinella PC. Attitudes of American adult women toward accepting RhD-mismatched transfusions in bleeding emergencies. Transfusion. 2022;62 Suppl 1:S211-S7.